
Why we need to continue breaking down the barriers to diagnosis
Ali lost her friend Jo and Aunt Carol to ovarian cancer, and urges change to be made.
Read our latest updates from research breakthroughs to government actions to fundraising heroes.

Ali lost her friend Jo and Aunt Carol to ovarian cancer, and urges change to be made.

Nedra, Georgia’s mum, was diagnosed with stage 4 ovarian cancer in December 2017 and sadly passed away two years later. Since 2018, Georgia has taken on a challenge every year to raise both awareness and funds for Target Ovarian Cancer.
The West End Sings for Target Ovarian Cancer –enjoy the music of Wicked, Phantom of the Opera, Les Miserables and much more!
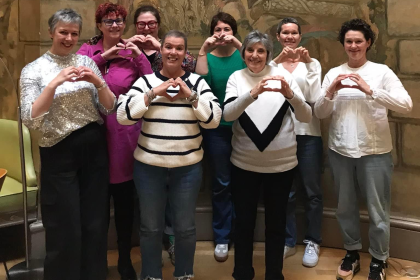
Treatment coming to an end can be a challenging time, often renewing a sense of isolation and uncertainty. For women with an ovarian cancer diagnosis, this can be exacerbated by a lack of connection to people who have had similar experiences.

We’re delighted to appoint Saswati Saha Mitra, Jo Paice and Angie Ma to the board leadership team to drive forward progress for those with ovarian cancer.

7,500 women are diagnosed with ovarian cancer every year. If diagnosed early, 9 in 10 will survive, however the reality is too many are diagnosed too late, and then just 2 in 10 are expected to survive five years or more.

We're delighted to be teaming back up with Olive and Frank for Ovarian Cancer Awareness Month 2024. This year, the ‘Open Heart’ hoodie has been created in addition to the tee!

Ovarian Cancer Awareness Month is in March. This year, we're more determined than ever to dispel common myths about ovarian cancer.

Find out more about the latest changes to our Board of Trustees, and hear from our new Chair.

Find out more about the impact of our campaigning work last year, and what we've got coming up for 2024!

We recognise the need for community and support for everyone impacted by ovarian cancer. We're sharing 5 ways to connect with others and find support.

Last summer we launched the Nurse Hero Awards – to recognise Clinical Specialist Nurses (CNSs) across the UK for their dedication and support to those living with ovarian cancer, and their families. We're delighted to announce the winners.
Sign up to receive emails and keep up to date on all things Target Ovarian Cancer
Sign up